What are enzymes?
Enzymes are bio-active organic molecules found in the cells of both prokaryotes and eukaryotes. Interestingly, enzymes are specific to a particular substrate. They contribute to specialized functions in all organisms for converting the substrate to a specific product through an enzymatic reaction.
Enzymes extracted from the biological system are mostly used for industrial purposes to produce valuable products and enhance both product quantity and quality.
Enzymes, for example, are used in the manufacture of sweeteners and the modification of antibiotic structure and functionality. Despite this, they are found in cleaning products such as washing powders, soaps, and other household cleaners. The main characteristics of enzymes are described below:
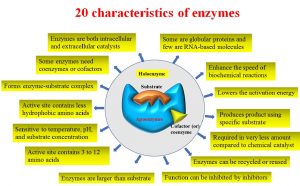
The main characteristics of enzymes include
- Enzymology is a subject that provides information related to enzymes’ basic principles, including structure, classification, kinetics, and inhibition, as well as their biological applications and purification procedures.
- Enzymes can be extracted from living cells and then used for industrial applications.
- There are two types of enzymes: simple and complex enzymes.
- Simple enzymes are only proteins, while complex enzymes are made up of proteins and cofactors.
- Most of the enzymes are globular proteins, and a few are RNA-based molecules.
- The RNA-based catalysts are called ribozymes, which are involved in gene expression along with protein-based enzymes.
- The RNA-based catalysts are called ribozymes, which are mainly involved in gene expression alongside typical enzymes.
- Enhance the speed of biochemical reactions by lowering the activation energy.
- The enzymatic reaction is very specific.
- produces the required product using the specific substrate.
- For biochemical reactions, enzymes are required in a very small amount compared to chemical catalysts.
- Enzymes can be recycled for further reactions.
- The enzymatic reaction can be inhibited by various inhibitors.
- Most of the enzymes are larger than the substrate, so the enzymes fit more tightly around the substrate.
- The active site in the enzyme structure is a very small portion that has between 3 and 12 amino acids.
- The function of enzymes depends on various factors, including temperature, pH, and substrate concentration.
- The chemical composition of the active site is important for accessing the substrate binding and controlling solvent access during the reaction.
- Enzyme active sites have some sort of charge, either negative or positive, hence termed a nucleophilic character.
- The active site contains fewer hydrophobic amino acids compared to entire proteins.
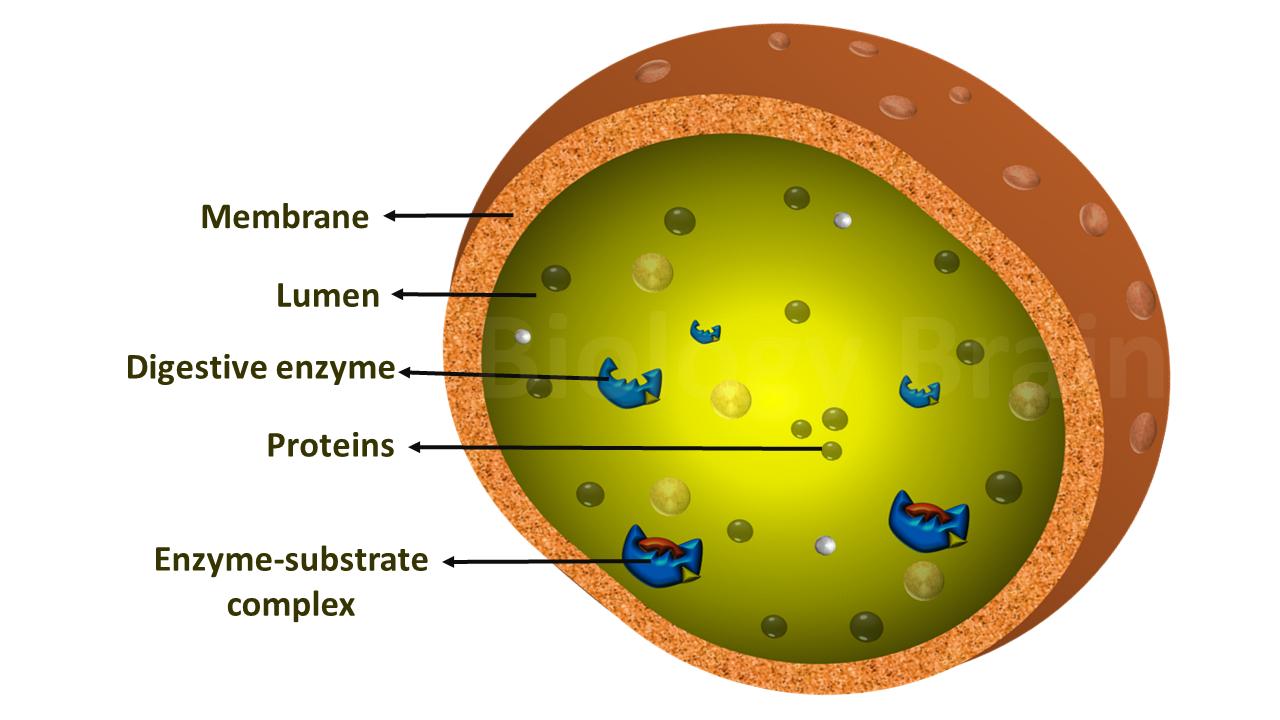
- The enzyme binds to its substrate and forms an enzyme-substrate complex.
- Some enzymes catalyze reversible reactions.
- Some enzymes need coenzymes or cofactors for the reaction to happen.
- Enzymes do not change the structure and function of final products once released from their active sites.
- Enzymes are both intracellular and extracellular catalysts.
- The enzymes’ catalytic activity can be expressed by a constant, kcat.
- The catalytic activity (kcat) is also referred to as the turnover rate, turnover frequency, or turnover number.
Enzyme specificity
- Enzymes typically show remarkable specificity, catalyzing reactions on only one type of substrate and producing similar products.
- Some enzymes, on the other hand, have group specificity regardless of substrate, meaning they will only act on substrates with specific functional groups.
- Alkaline phosphatase, for example, is capable of removing phosphate groups from a wide range of substrates.
- Moreover, other enzymes have much higher absolute specificity, which is a term used to describe how specific they are. Glucose oxidase, for instance, is almost completely specific for its molecule, β-D-glucose, and has little activity with other monosaccharide molecules.
- The absolute specificity of an enzyme is crucial in many analytical methods and devices (biosensors) that analyze a distinct substrate molecule or analyte (e.g. lactate) in a complex mixture (e.g. bacterial culture).
References:
- The Central Role of Enzymes as Biological Catalysts. The Cell: A Molecular Approach. 2nd edition. (https://www.ncbi.nlm.nih.gov/books/NBK9921/)
- Poonam Singh Nigam. Microbial Enzymes with Special Characteristics for Biotechnological Applications. Biomolecules, PMC4030947.
- Peter K. Robinson. Enzymes: principles and biotechnological applications. Essays Biochem. doi: 10.1042/bse0590001.
- Enzymes. Br J Pharmacol, 10.1111/j.1476-5381.2011.01649_9.x.
- A. C. Peterson and M. F. Gunderson. Some Characteristics of Proteolytic Enzymes from Pseudomonas fluorescens. Appl Microbiol, PMC1057578