Definition of activation energy: Some amount of energy must be put into get the reaction initiated. This energy is required to activate the substances to react. Hence, this energy is called activation energy.
In the initial step of the reaction, enzymes by functioning as catalysts reduce the activation energy required for a chemical reaction to take place. Without altering the process, enzymes speed up the overall rate of reaction.
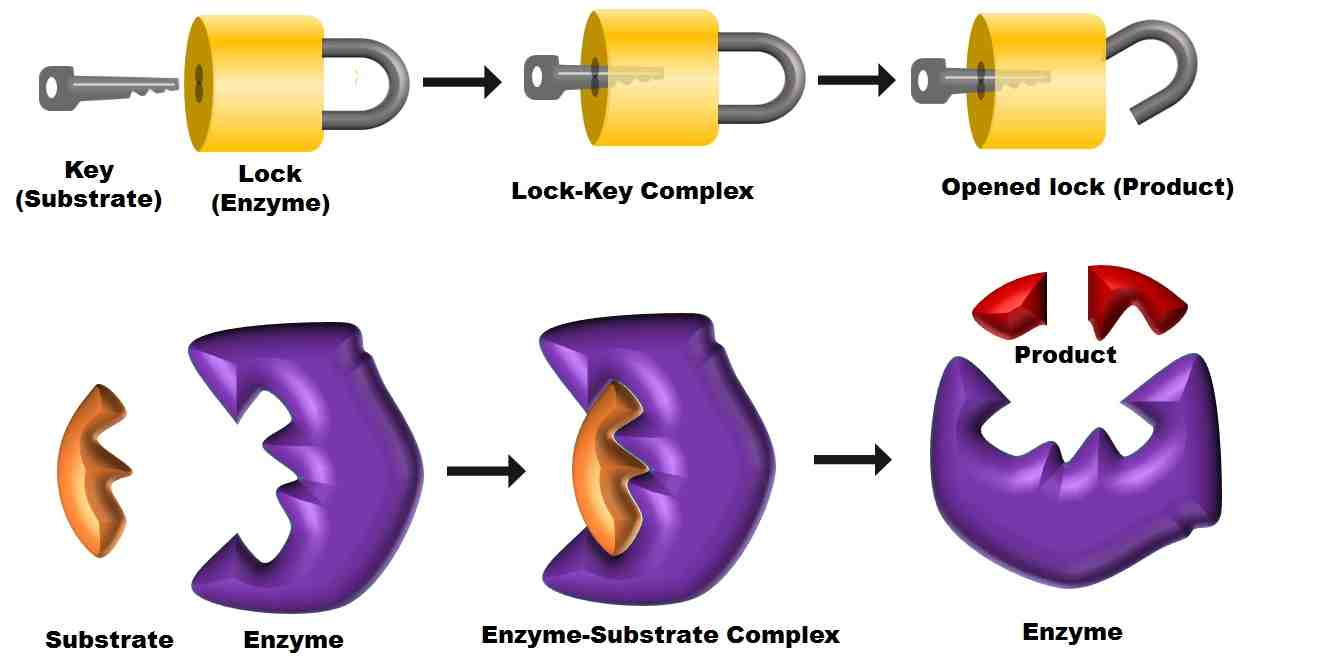
lock and key hypothesis of enzyme action
- Enzymes are very specific to the substrates.
- Lock and key hypothesis was proposed by Emil Fischer in 1890.
- The specificity of an enzyme towards substrate is significant because enzymes have a particular shapes, into which substrate fit exactly.
- The substrate is imagined as being like a key whose shape is complementary to the lock (the enzyme shape).
- The site where the substrate binds to the enzyme is known as the active site, which has a specific shape.
The enzyme catalytic process has been described in the following stages.
- Initially, the substrate binds to the active site of an enzyme, fitting into an active site.
- The binding of the substrate induces the enzyme to alter its shape and that leads to fitting more tightly around the substrate.
- The active site of an enzyme breaks the chemical bonds of the substrate and forms new enzyme-product complex.
- After the reaction, the enzyme releases the final products by decreasing its affinity towards its product.
- Finally, the enzyme will be ready again to involve in another enzymatic reaction , which means that the enzymes can recycle the process by binding the other substrate molecules.
Lock and key hypothesis diagram
- Mostly, molecular structures of the enzymes are far larger than reactants (substrate) they act on.
- However, the active site in the enzyme structure is a very small portion having between 3 to 12 amino acids.
- The remaining portion of the enzymes has around 20 to 200 and more amino acids, which make up the enzyme bulky and maintain the functioning portion (shape) of the enzymes correctly.
- This extra part of the enzyme is also very important if the active site is to function at the maximum rate.
- Once the enzyme converts the substrate to product, the product no longer fits into the active site, immediately releases into the surrounding medium.
- Leaving the active site free, welcomes the other substrate molecule to fit into it, and that makes the next enzymatic reactions to take place.
Factors affecting the mechanism of enzyme action
- Enzymes are mostly proteins (except some RNA-based enzymes) with tertiary structures.
- Any alterations in the enzyme structure would affect the overall activity of the enzymatic reaction.
- Some of the abiotic factors can directly affect the enzymatic reaction.
(i) Hydrogen ion concentration on the mechanism of enzyme action
- The concentration of hydrogen ions (pH) during enzymatic reactions plays a crucial role.
- An enzyme at certain pH shows a maximum activity, which is known as the optimum pH of an enzyme.
- Increasing or decreasing pH will decline the overall activity of an enzyme. Some enzymes function well in an acidic medium, others in an alkaline medium.
(ii) Temperature on the mechanism of enzyme action
- Enzymes are typically activated (function) at a certain range of temperature; however, an optimum temperature for reaction may be various from enzyme to enzyme depends on their origin.
Example:- Taq polymerase is a heat stable DNA polymerase synthesized by thermophilic bacterium Thermus aquaticus. In research labs, the name of the enzyme termed TaqPol or simply Taq. This enzyme can replicate DNA even at above 90°C. The half-life of Taq is more than 2 hours at 92.5°C.
- The enzymes show the maximum rate of enzymatic reaction at certain temperature is known as optimum temperature.
- Which means enzymatic reaction declines at both below and above optimum temperature.
- At low-temperature state, enzymes are temporarily inactive. When the temperature rises to a normal state then the enzymes will gain their lost activity. This low-temperature state is generally used in laboratory conditions to preserve the enzymes for a longer period.
- Higher temperature is an enemy for most of the enzymes. At this state, enzymes are denatured and lost their activity by destroying the weak hydrogen bonds of their tertiary structure.
- These structural changes make the enzymes lost their catalytic activity. Once the enzyme (protein) is denatured, then it remains inactive even if the temperature comes back to a normal state.
(iii) Substrate concentration on the mechanism of enzyme action
- The concentration of substrate in the enzymatic reaction decides overall rate of reaction.
- An increase in substrate concentration increases the velocity of the reaction.
- At a certain stage, the rate of reaction ultimately reaches a maximum velocity (Vmax).
- At this point, there is no exceeded rate of reaction even if further raises in substrate concentration.
- This is because all the enzyme molecules are completely saturated with the substrate under this conditions.
- That means there is no active site for the binding of additional substrate molecules.
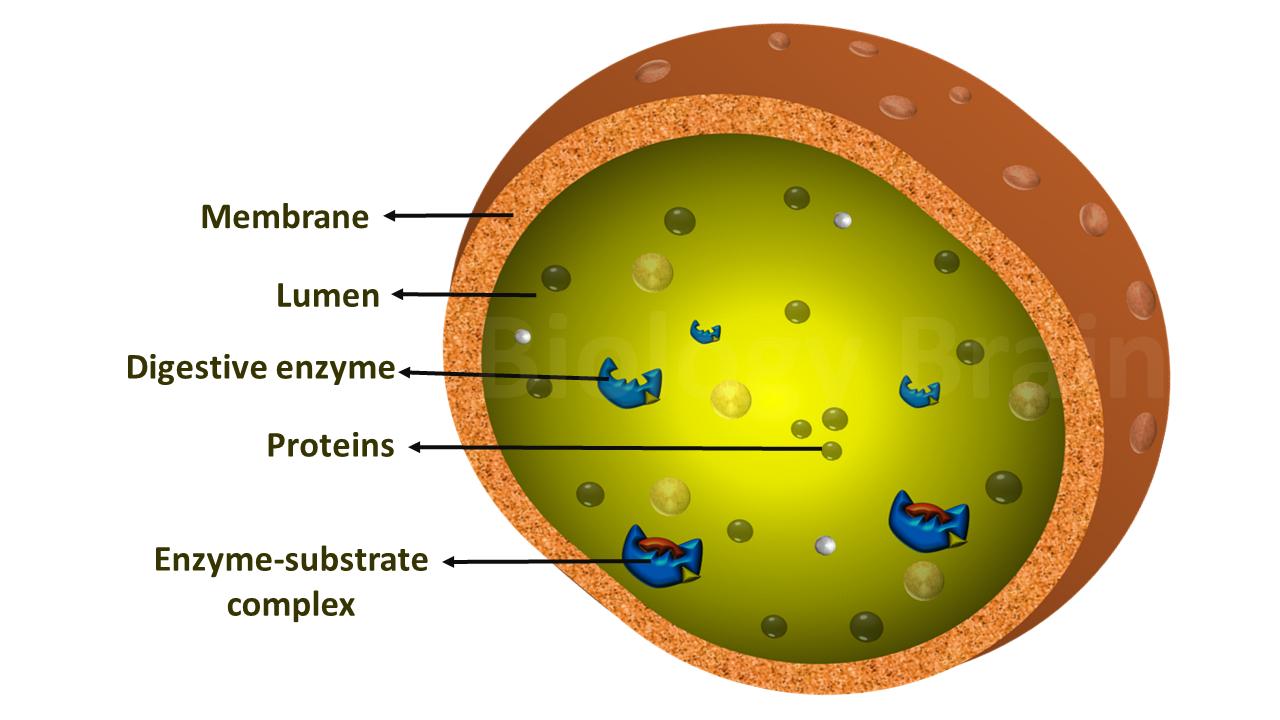
Components of the enzyme active site
- The dynamic changes and motions of the enzyme active site are very crucial during the catalytic process.
- The chemical composition of the active site is important to access the substrate binding and for controlling solvent access during the reaction.
- Active sites of the enzymes have some sort of electrical charge, that will be either negative or positive, termed as a nucleophilic character.
- The nucleophilic character of the active site with any charge is developed by side chains of the amino acids present in the active site.
- Hydrophobic side chain containing amino acids such as Ala, Val, Leu, Ile are found less frequently in the active sites than that in the rest of the protein.
- While aromatic side chain containing amino acids (Trp, Tyr, and Phe), small polar side chain containing amino acids (Ser, Thr), and especially the smallest amino acid (Glycine) are found frequently in the active sites of an enzyme.
- The charges of the active site allow the enzymes to bind with specific substrates to react (oxidation or reduction).
- Allowing the substrate and any solvent into the active site of the enzyme is strictly controlled by gates found either in the active site or in front of the catalytic cavity.
- The gates present in the active site contain amino acid side chains, which close or open the access pathway by rotating themselves.
- For example, in some cases exclusion of water from some parts of the cavity, such as the active site or a specific tunnel of the enzyme, is necessary for the functioning of numerous enzymes.
- In most of the enzymes, gates prevent the entering of solvent and protonation of the ammonia present in the active site, which is very crucial to access the substrate binding.
- In the enzyme cytochromes P450, the gate (water channel) controls the hydration of the substrate in the active site, which is most important for cytochrome activity.
- Interestingly, in some enzymes, the gates may permit the solvent to enter only to a specific part of the cavity, e.g. carbamoyl phosphate synthetase.
- In the enzyme hydroxysteroid dehydrogenase, the gates may prevent the access of water molecules into the cavity when a substrate or a cofactor is not found.
- Gates also play a major role in filtering the potential substrate from the non-specific substrate, thus they play a crucial role in controlling the selection of certain substrate.
Some examples of enzymes which are having gates at the entrance of their active sites, including……
- Imidazole glycerol phosphate synthase
- Monooxygenase
- Toluene-o-xylene monooxygenase
- Acetylcholinesterase
- Type III polyketide synthases
- Choline oxidase
- NiFe hydrogenases
- Carbonic anhydrases
- Formiminotransferase-cyclodeaminase
- Glutamate synthase
- FabZ β-hydroxy–acyl carrier protein dehydratase
Related post: The major characteristics of enzymes.
Michaelis constant
Michaelis constant (Km) is a mathematical derivation or constant which describes the substrate concentration at which the chemical reaction catalyzed by an enzyme attains half of enzyme maximum velocity or the concentration of the substrate at which half the maximum velocity of the enzyme reaction is attained.
References:
- Ashutosh Tripathi and Vytas A Bankaitis. “Molecular Docking: From Lock and Key to Combination Lock.” Journal of molecular medicine and clinical applications vol. 2,1 (2017).
- Emmanuelle Schmit, Caglar Tanrikulu, Tae Hyeon Yoo, Michel Panvert, David A. Tirrell, and Yves Mechulam. Schmitt, Emmanuelle et al. “Switching from an induced-fit to a lock-and-key mechanism in an aminoacyl-tRNA synthetase with modified specificity.” Journal of molecular biology vol. 394,5 (2009).
- Hans-Jörg Schneider. “Limitations and extensions of the lock-and-key principle: differences between gas state, solution and solid state structures.” International journal of molecular sciences vol. 16,4 6694-717. 2015.
- Dror Tobi and Ivet Bahar. “Structural changes involved in protein binding correlate with intrinsic motions of proteins in the unbound state.” PNAS. vol. 102,52 (2005): 18908-13.
- Pratul K Agarwal. “Enzymes: An integrated view of structure, dynamics and function.” Microbial cell factories vol. 5 2. 12 Jan. 2006, doi:10.1186/1475-2859-5-2.