Isoenzymes Definition
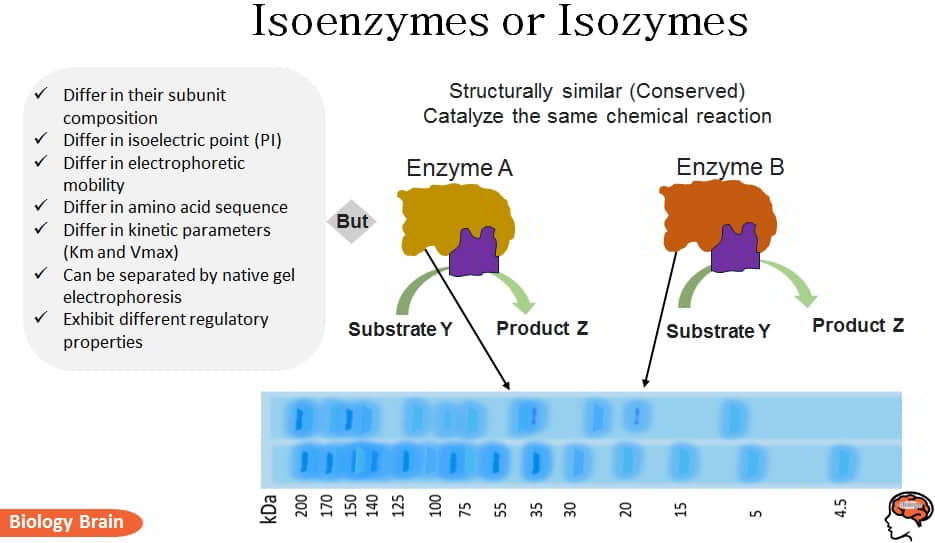
Isoenzymes or isozymes are different versions of the same enzyme that catalyze the same biochemical reactions. Different alleles of the same gene encode each isozyme for a given enzyme. Different isozymes have different amino acid sequences, isoelectric points (PI), electrophoretic mobility, and kinetic parameters (Km and Vmax).
The isoenzymes can be easily divided using native gel electrophoresis and ion-exchange chromatography because they differ in their subunit composition and isoelectric point.
The isozymes frequently exhibit variations in their kinetic properties because they were developed to meet the needs of various tissues.
“Isozymes” come from different genetic forms. For instance, human alkaline phosphatases, which have at least three distinct genetic sources, including kidney, liver, bone, placental, and intestinal enzymes, are a good illustration of isoenzymes.
What are Isoenzymes or Isozymes and Examples?
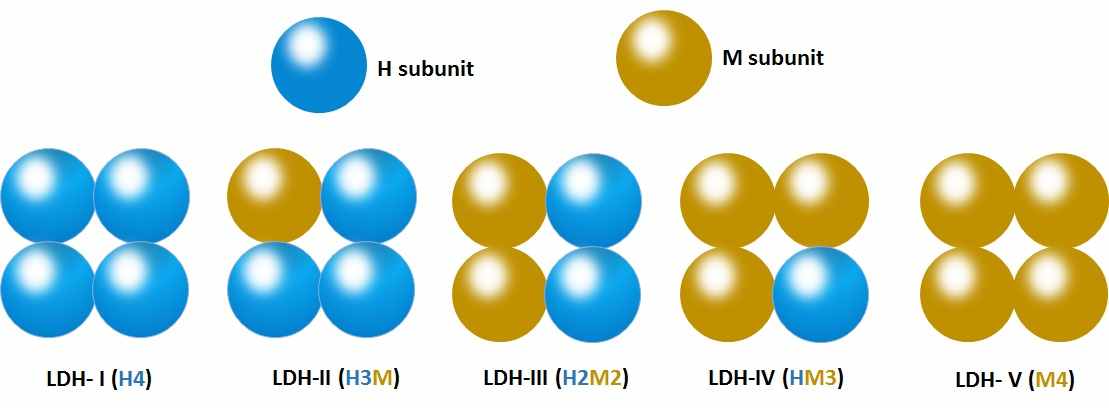
The well-studied isozymes are those of lactate dehydrogenase (LDH), the enzymes that are responsible for the reduction of pyruvate to lactate.
LDH is a tetrameric enzyme that can be found in homotetrameric or heterotetrameric forms.
There are five possible isozymes of LDH that have been studied.
a) LDH- I (H4) is a homotetramer of ‘H’ subunits; present in heart cells.
b) LDH-II (H3M), LDH-III (H2M2), and LDH-IV (HM3) are tetramers distributed in different tissues.
c) LDH- V (M4) is a homotetramer of ‘M’ subunits present in skeletal muscle cells.
Very high Km for substrate and very low affinities and Vmax are characteristics of the heart form of LDH. These properties are very common for heart form since pyruvate accumulation rarely occurs due to the aerobic nature of heart muscle cells.
The form of skeletal muscle has an extremely low Km, a high Vmax, and a very high affinity for the substrate.
Skeletal muscle has undergone extensive research for the speedy conversion of pyruvate to lactate. Due to the skeletal muscle cells’ dependence on anaerobic respiration, this conversion is absolutely essential.
Muscle fatigue is brought on by an accumulation of lactate caused by increased LDH activity in skeletal muscle cells.
Isozymes of LDH are also used in clinical biochemistry as diagnostic markers. LDH-I is a marker for myocardial infection, while LDH-V is a marker enzyme for muscular dystrophy.
Characteristics of Isoenzymes
- Isoenzymes, also referred to as isozymes, are distinct forms of the same enzyme activity that take place in various tissue ratios.
- It appears that multiple molecular forms (isoenzymes) of some enzymes are necessary for all living systems to reach their full biological potential.
- The composition, sequence, and multimeric quaternary structure of isoenzymes vary; generally, but not always, they have similar (conserved) structures.
- Numerous investigations revealed that the two isozymes were synthesized from two distinct mRNA molecules.
- The control of the gene for each subunit determines how they are expressed in a particular tissue.
- The emergence of isozymes is caused by gene duplications and/or different epigenetic modifications to a gene product (or gene products).
- Isozymes are, in this sense, the majority of recombinant enzymes with deletion, insertion, and/or other genetic mutations.
- Some isoenzymes are tissue or organ-specific.
- According to its function in that tissue, each isoenzyme form will have unique kinetic and/or regulatory characteristics.
- The majority of the isoenzymes for a given enzymatic function turned out to have identical amino acid sequences in their active site, with a few exceptions.
- Even though isoenzymes have the same enzymatic function, they differ in their enzyme forms and catalytic efficiencies.
- Additionally, they show various electrophoretic mobilities.
- Hence, typically, electrophoresis is used in clinical laboratories to identify isoenzymes.
- The same organism produced both of these isoenzymes easily distinguishable forms of an enzyme.
- Isozymes, despite catalyzing the same chemical reaction, typically differ in terms of their fundamental makeup, intracellular location, and physiological role.
- For instance, animals’ produced HMG-CoA synthase isozymes are referred to as mitochondrial or cytosolic isozymes depending on where they are found within the cell.
- Instead of being synthesized by differential splicing of a single gene product, these are thought to be the byproducts of various genes.
- Isozymes can also differ in terms of their kinetic characteristics. The cytosolic HMG-CoA synthase, for instance, is stimulated by Mg2+, whereas the mitochondrial HMG-CoA synthase is inhibited by Mg2+
- However, some studies have discovered that some isoenzymes exhibit the opposite function. For instance, 11-hydroxysteroid dehydrogenase is essential for the metabolic process. This enzyme’s two isozymes are essential for the synthesis of glucocorticoids.
- The primary isozyme 11beta-HSD type 1 (11-HSD1) is primarily responsible for catalyzing the conversion of inactive cortisone to active cortisone in healthy cells and organs.
- While its secondary isozyme called 11-HSD type 2 (11-HSD2) is responsible for the quick dehydrogenation of active cortisol into inactive cortisone (11-dehydrocorticosterone).
- Overall, these two isozymes collectively have the potential to be crucial in glucocorticoid feedback.
Frequently asked questions
Q Which is the following about isoenzymes is correct
- Isozymes are the same in their subunit composition and catalyze the same biochemical reaction.
- Isoenzymes have the same isoelectric point and catalyze different biochemical reactions.
- Isoenzymes have different amino acid sequences and catalyze different biochemical reactions.
- Isoenzymes differ in kinetic parameters and show different catalytic activity.
Answer: 4 (Isoenzymes differ in kinetic parameters and show different catalytic activity).
Q Isoenzymes be separated by
- Column chromatography and Ion exchange chromatography.
- Gel electrophoresis and Ion exchange chromatography.
- Gel electrophoresis and Column chromatography.
- None of the above.
Answer: 2 (Gel electrophoresis and Ion exchange chromatography).
References
- John W.Pelley, 2012. 4 – Enzymes and Energetics (doi.org/10.1016/B978-0-323-07446-9.00004-0).
- Daniel A.BocharJona.FreisenCynthia V.StauffacherVictor W.Rodwell, 1999. 2.02 – Biosynthesis of Mevalonic Acid from Acetyl-CoA. (doi.org/10.1016/B978-0-08-091283-7.00035-7).
- GeorgeFink, 2012. Chapter 3 – Neuroendocrine Feedback Control Systems: An Introduction (doi.org/10.1016/B978-0-12-375097-6.10003-4).
- Paschal A.Oude WeerninkGerard E.J.StoalGertRijksen, 2002. Pyruvate Kinases (doi.org/10.1016/B0-12-227555-1/00518-9).
- Hyone-MyongEun, 1996. 1 – Enzymes and Nucleic Acids: General Principles (doi.org/10.1016/B978-012243740-3/50004-1).