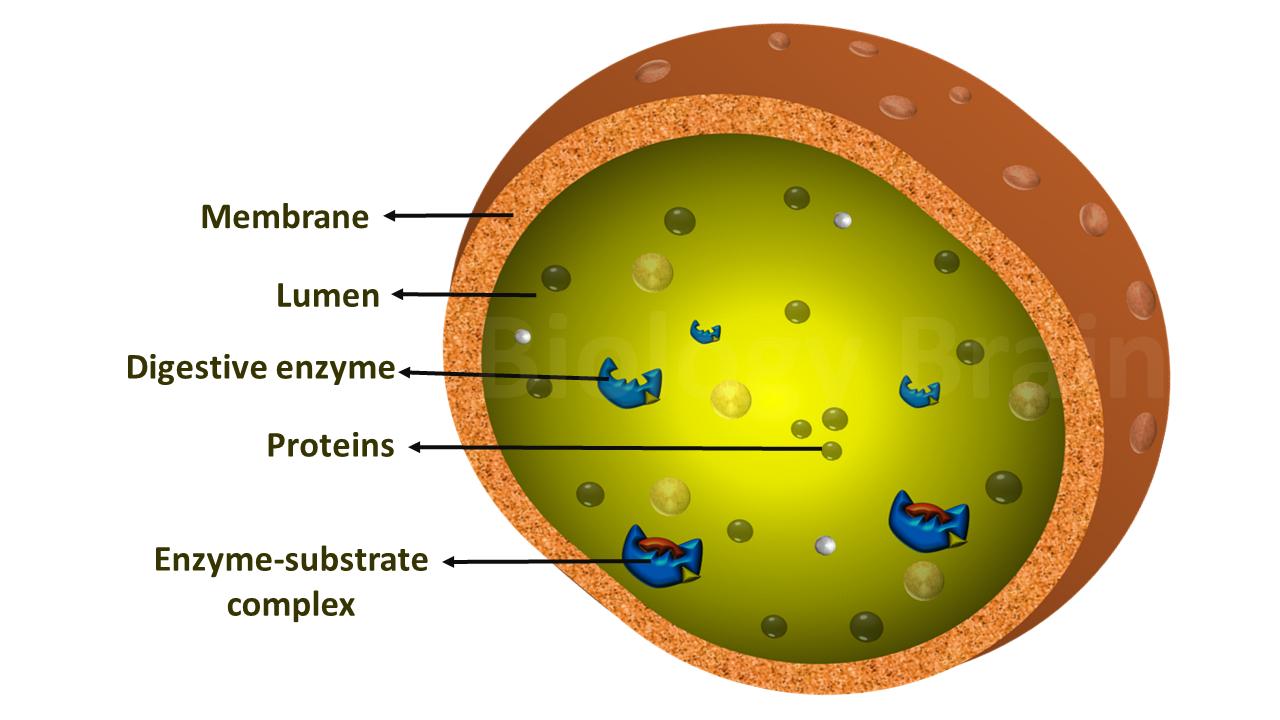
Complex enzymes are made up of protein and nonprotein components. The protein part of the enzyme is called Apoenzymes or Apozyme. While the non-protein part of the enzyme is called Cofactor.
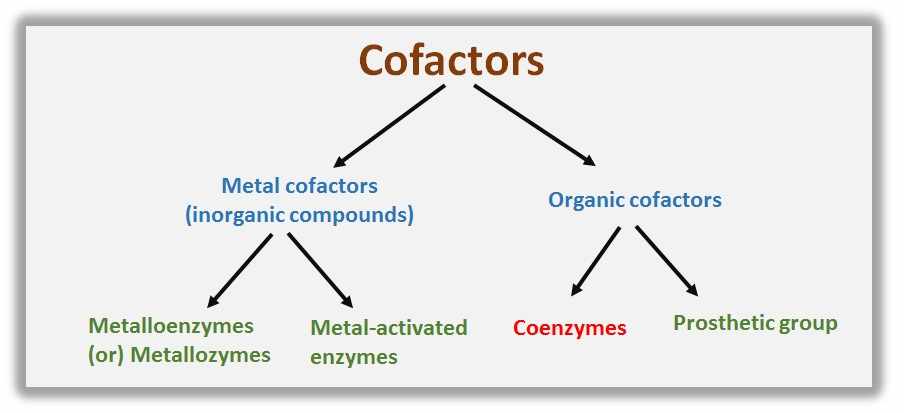
Cofactors are two types based on their chemical properties such as…..
1. Metal cofactors (inorganic compounds)- Metalloenzymes (Metallozymes) and Metal-activated enzymes
2. Organic cofactors – Coenzymes and Prosthetic groups
Unlike complex enzymes, a simple enzyme can be made up of only protein components and they can complete the enzymatic reaction alone without a cofactor.
The combination of apoenzyme and cofactor together is called the Holoenzyme.
The intact holoenzyme is biologically active and contains catalytic activity.
Alone apoenzyme and cofactor will not be biologically active, hence they cannot fulfill the enzymatic reactions.
Metal cofactors
1. Metalloenzymes or Metallozymes
Enzymes having the transition metal ions as cofactor are called metalloenzymes or metallozymes.
The transition metal ion can participate in the formation of an enzyme active site which is a place for substrate binding.
The shape of the active site in the enzyme is crucial and once the transition metal binds to the enzyme then it will be always bound to the active site.
Examples of Cofactors (metalloenzymes)
Zn2+ is the most common metal ion found in the enzyme Alcohol dehydrogenase.
The dinitrogenase complex is one of the metalloenzymes involves in the biological nitrogen fixation (BNF) containing more than one transitional metal ion, dinitrogenase contains both Mo and Fe.
2. Metal-activated enzymes
Some enzymes that require alkali or alkaline earth metal ions are called metal-activated enzymes.
In such enzymes, the metal ion requires only at the time of reaction to activate the enzymes or to complex with the substrate.
Once the enzyme-substrate complex formed or reaction completed then the metal will be separated from the enzyme, whereas these ions will not take part in the enzyme active site formation.
Organic cofactors
The organic cofactors are derivatives of the B-complex group of vitamins.
These are further divided into coenzymes and prosthetic groups.
1. Coenzymes
Coenzymes are organic cofactors that are loosely bound to the enzymes and form the active site. Coenzymes can bind the enzyme only at the time of reaction.
In few cases, coenzymes act as co-substrate and can be removed from the enzyme without damaging the structure of an enzyme.
Interestingly, coenzymes themselves exhibit poor catalytic properties compared to enzyme-coenzyme complexes (1).
Example of coenzyme (organic cofactors):
1. Pyridoxal phosphate is the coenzyme that is capable of catalysis even in the absence of apoenzyme (protein part) even though the catalytic activity is relatively low compared to the Holoenzyme.
2. The pyridine nucleotides (NAD+ and NADP+) and both NAD and NADP play crucial roles in pro-oxidant and antioxidant metabolism. Pyridine nucleotides are involved in many other defense and signaling reactions, for example, the production of nitric oxide and the metabolism of reactive lipid derivatives. NAD and NADP are also used as respective substrates for the production of the calcium agonists cyclic ADP-ribose and nicotinate adenine dinucleotide phosphate.
3. Co-enzyme A is a cofactor involved in the fatty acid synthesis and oxidation and also involved in the oxidation of pyruvate in the citric acid cycle.
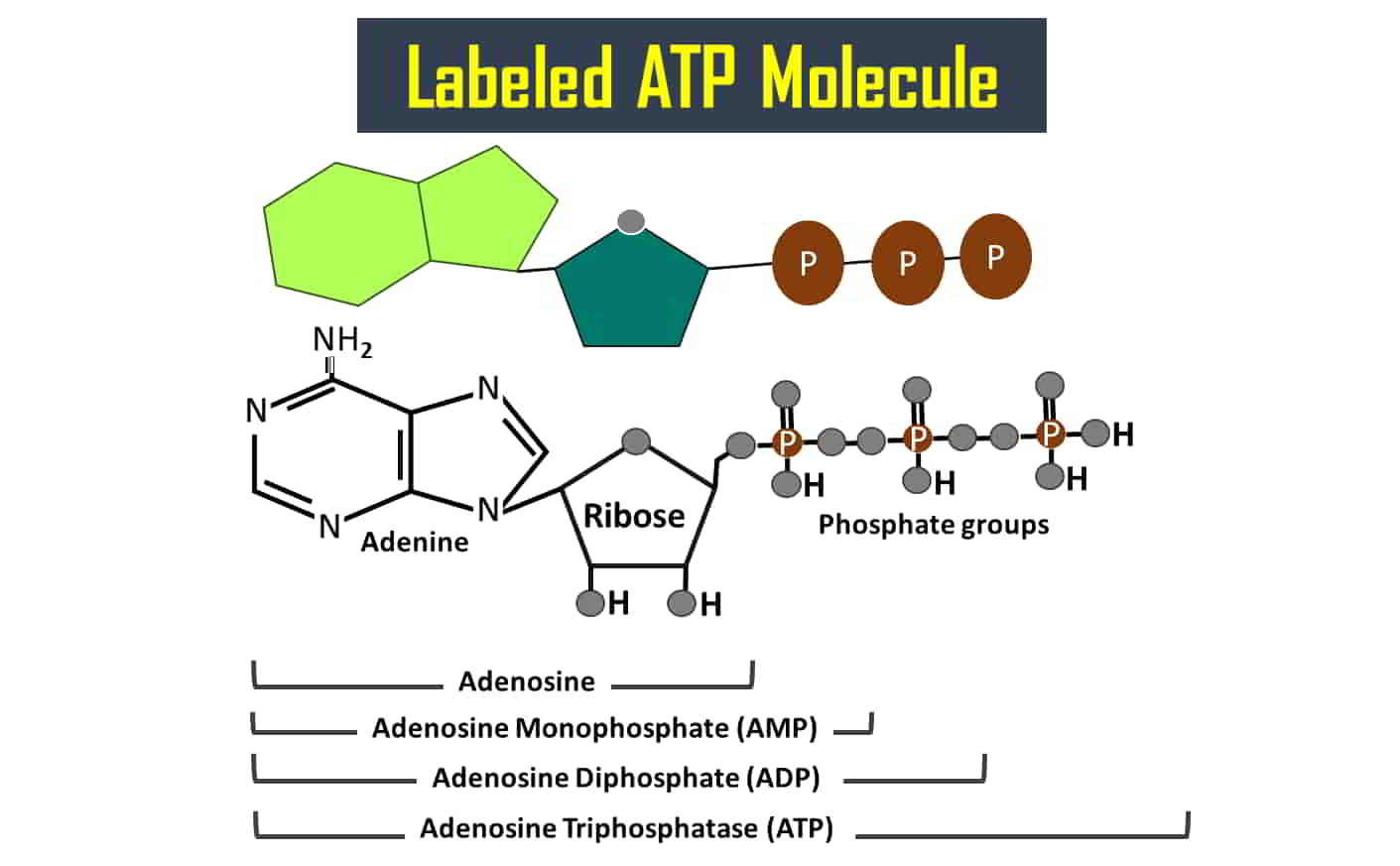
4. ATP is one of the coenzymes involved in different types of enzymatic reactions as a cofactor.
2. A prosthetic group
A prosthetic group is an organic cofactor that is tightly bound to the enzyme and involved in active site formation.
The prosthetic groups may be organic compounds such as a vitamin, sugar, or lipid, but the prosthetic group is not composed of amino acids.
Like metal ion cofactors, once the organic cofactors bind to the enzyme then they always remain with the enzyme complex.
The prosthetic group cannot be removed from the enzyme until the structure of the enzyme gets denatured.
Examples of Cofactors (organic prosthetic groups)
1. Biotin: ATP-dependent carboxylation enzymes such as pyruvate carboxylase, acetyl Co.A carboxylase, and propionyl Co. A are contain biotin [vitamin H] as the prosthetic group.
Avidin is a tetrameric biotin-binding protein synthesized in the oviducts of birds, reptiles, and amphibians and then deposited in the whites of their eggs. The extremely high affinity of biotin to the enzymes (e.g. avidin) makes it a potent inhibitor of biotin-dependent enzymes. The binding of biotin to avidin leads to defects in carbohydrate metabolism (gluconeogenesis) and lipid biosynthesis.
2. FMN and FAD: The flavin nucleotides (FMN and FAD) are prosthetic groups that regulate the activity of the oxidoreductase enzyme, which catalyzes the transfer of electrons from one molecule, the reductant (electron donor) to another molecule, the oxidant (electron acceptor) in the electron transfer system.
Enzyme inhibitors
Some of the metals can inhibit the overall enzymatic reaction
Pb2+, Hg2+, Hg+, and Co2+ metals are the best examples of enzyme inhibitors.
The strong metal inhibitors Pb2+ and Hg2+ are binding with the cysteinyl SH group of the enzyme and removes the catalytic activity of enzymes.
References:
1. Prof. Dr. Andreas Kirschning. Coenzymes and Their Role in the Evolution of Life. Wiley online library.
Read More