Anfinsen’s experiments indicate that the formation of higher-order structures is mainly dependent on the nature and type of amino acids present in the protein’s primary structure.
Anfinsen’s experiment in protein folding used the following components:
- RNase enzyme (experimental enzyme)
- β-mercaptoethanol (denaturation agent)
- Urea solution (denaturing agents)
- Different dialysis fluids
Anfinsen’s experiment conclusion
In his experiments, he performed the denaturation and renaturation processes using the enzyme, pancreatic ribonuclease as the experimental protein. RNAse is an extracellular enzyme that possesses a single subunit that contains eight cysteine residues involved in the formation of four disulfide bridges.
The tertiary structure of RNase is stabilized by the formation of both covalent and noncovalent interactions. He measured the biological activity of the native RNase before the experiment and then subjected the RNase enzyme to denaturation using β-mercaptoethanol and urea as denaturing agents.
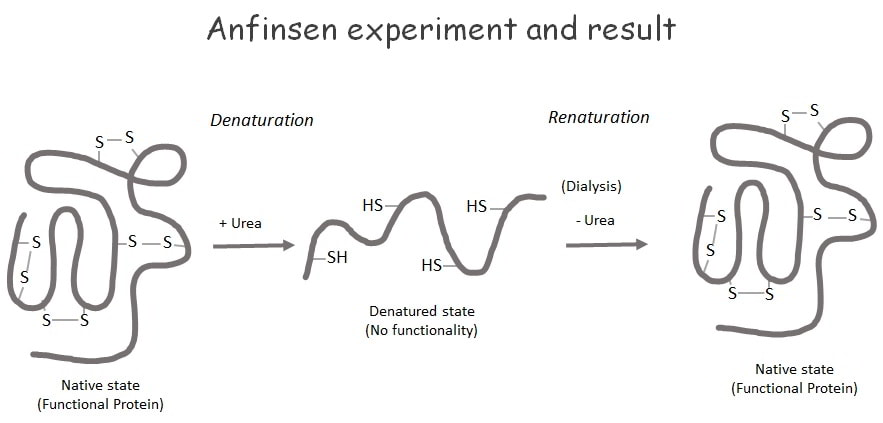
The denaturation process removes the biological activity of the enzyme. When Anfinson renatured the denatured proteins using the method of dialysis, he found the regaining of biological activity in denatured proteins. It states that the process of dialysis can remove all low molecular-weight denaturing compounds, such as urea, β-mercaptoethanol, etc. The main intention of his experiment is to prove that the information in an amino acid sequence of a protein is important for protein folding during tertiary structure formation.
Some exceptions to the Anfinsen experiment include:
- All proteins cannot go through spontaneous refolding as the RNase enzyme.
- For some of the proteins, the folding process will be completed by chaperons, which are a group of heat shock proteins (HSP) proteins that facilitate protein folding and subunit-subunit interactions. One of the mechanisms of chaperons is to facilitate protein folding by bringing hydrophobic side chains together to form a nucleation center.
Noble prize and applications of his principles
Christian B. Anfinsen was awarded the Nobel Prize in Chemistry in 1972 for this work.
In this study, he discovered a link between the amino acid sequence and the biologically active conformation of proteins.
Overall, the protein folding studies, bio-computing sciences, and protein design approaches are all based on these well-established basic links, so understanding these principles is essential for current researchers.
These experimental principles give students the tools they need to interpret the structural and kinetic experiments designed to help them understand the protein folding problem on their own.
Furthermore, this simple experiment also allows students to manipulate proteins, develop new biochemical approaches, and conduct spectroscopic and chromatographic techniques.
The thermodynamically spontaneous process in protein folding indicates the changes in negative free energy [∆G<0].
∆G= ∆H-T∆S
∆G for a process depends on ∆H, ∆S, and temperature [T]
∆G= Free energy change
∆H= enthalpy change
∆S= entropy change
Protein folding is a biological process in the living cell, that does not depend on the temperature.
So, in the process of protein folding, there is no change in temperature since ∆H and ∆S will be the major contributors to the free energy change.
The entropy change has two components
1) Change in the entropy of the protein.
2) Change in the arrangement of water molecules around the protein.
If protein folding converts highly randomly coiled proteins into a highly organized, compact form, then the entropy will decrease and, therefore, it will have a negative contribution to the folding.
In the case of the water molecule, the protein folding increases the randomness of well-organized water molecules that are surrounded by the randomly coiled protein. Disruption of the well-organized water molecule is required for protein folding to take place.
Hence, the disruption of water molecules will have a positive contribution to protein folding. Thus, the overall entropy change will be negligible and it will not contribute to negative free energy change. Hence, changes in the enthalpy of the protein [∆H] will be the major contributor to the negative free energy change.
As the protein folding occurs, the energy content of the protein folding decreases. Therefore, ∆H becomes negative, which in turn makes ∆G negative, and folding becomes spontaneous.
References:
Andres Fernandez-Reche, et al. Approaching the thermodynamic view of protein folding through the reproduction of Anfinsen’s experiment by undergraduate physical biochemistry students. Biochem Mol Biol Educ. 46(3):262-269
Staffan Normark. Anfinsen comes out of the cage during assembly of the bacterial pilus. Proc Natl Acad Sci U S A. 97(14): 7670–7672.
C B Anfinsen, H A Scheraga. Experimental and theoretical aspects of protein folding. Adv Protein Chem. 1975;29:205-300.