Metal ions are important factors in cellular metabolism, as they function as enzyme cofactors and thus modulate the activity of enzymes and enzymatic reactions. Although several enzymes have been reported to interact with metal ions, the quantitative relationships between metal ions and metabolic reactions are needed to be analyzed. Here, some of the metal ions and their role in metabolic reactions with their respective enzymes have been tabulated.
Role of Metal Ions in Biological Systems as cofactors
| S.No | Metal Ion | Enzyme and its function |
| 1 | Zn2+
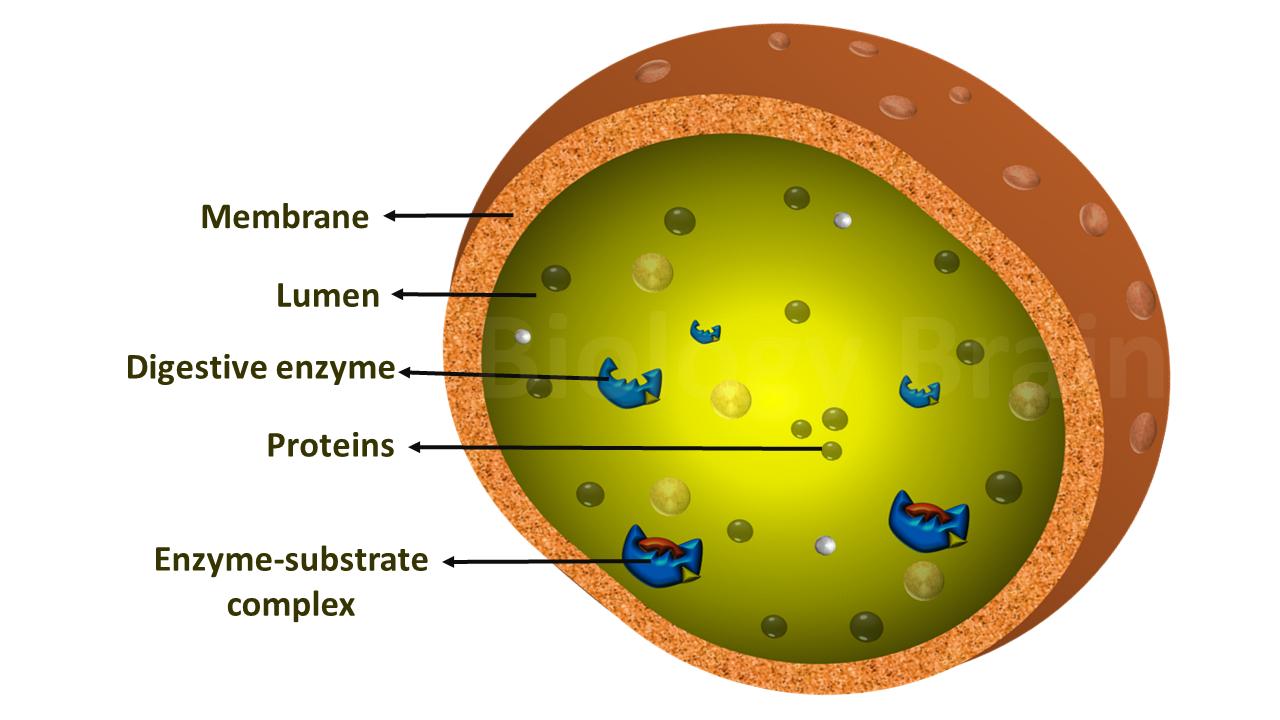
| Alcohol dehydrogenase: ADH catalyzes the alcohol into acetaldehyde with the reduction of nicotinamide adenine dinucleotide (NADH). Further, the aldehyde dehydrogenase (ALDH) enzyme converts acetaldehyde into acetate. |
| Carbonic anhydrase: This enzyme is present in red blood cells, which balances the blood pH and enables the breathing out of Co2 by converting the Co2 into carbonic acid, which further breaks down into bicarbonate ions and protons (H+). | ||
| Carboxy peptidase: It hydrolyzes a peptide bond at the carboxyl end (C-terminal) of a protein or peptide with the aromatic or aliphatic side-chains | ||
| Aminopeptidase: It is distributed throughout the animal and plant kingdoms and hydrolyzes a peptide bond at the amino end (N-terminal) of a protein or peptide. | ||
| IAA oxidase: It regulates the abscission in plants by auxin destruction. | ||
| 2 | Mo2+ | Dinitrogenase complex: These enzymes are produced by cyanobacteria and responsible for the reduction of nitrogen to ammonia. |
| Nitrate reductase: This enzyme fertilizes the soil by reduction of nitrate to nitrite. This reaction is very important for the production of nitrites because nitrites are the main source of protein synthesis in plants. | ||
| DMSO- reductase: This enzyme produces by certain bacteria such as purple bacteria and that catalyzes the reduction of dimethyl sulfoxide to dimethyl sulfide. | ||
| Xanthine oxidase: This enzyme generates reactive oxygen species and oxidizes the hypoxanthine to xanthine, further oxidizes the xanthine to uric. | ||
| 3 | Mg2+ | Kinase: This enzyme is involved in the phosphorylation process in which the higher energy phosphate group will be transferred to the substrate. |
| RuBisCO: The enzyme Ribulose-1,5-bisphosphate carboxylase/oxygenase present in plants and involved in carbon fixation, in which atmospheric carbon dioxide is converted to high-energy molecules such as glucose. | ||
| PEP carboxylase: The enzyme Phosphoenolpyruvate carboxylase is present in plants and some bacteria that are involved in the formation of the four-carbon compound, oxaloacetate. | ||
| DNA polymerase: The enzyme DNA polymerase is involved in the DNA replication of prokaryotes and eukaryotes using deoxyribonucleotides. | ||
| DNA ligase: This enzyme joins DNA molecules together by forming the phosphodiester bond. | ||
| Restriction enzyme: This enzyme is also called a restriction endonuclease that breaks DNA into fragments at specific recognition sites present within DNA molecules known as restriction sites. | ||
| 4 | Ca2+ | Calpains: The enzyme also known as non-lysosomal cysteine proteases (proteolytic enzymes) present in many organisms and responsible for many regulatory functions such as cell mobility and cell cycle progression regulate clotting, the dimension of blood vessels, playing a role in memory, skeletal muscle protein breakdown, apoptotic cell death and necrosis. |
| Calcineurin: Calcineurin is a Ser-Thr protein phosphatase regulated by both Ca2+ and calmodulin, that is crucial for the translation of Ca2+ signals into changes in cell function and development. In the mammalian immune system, calcineurin is crucial for T-cell activation. In organ transplantation, immunosuppressant drugs such as cyclosporine will be used to inhibit the function of calcineurin. | ||
| Ca2+– Calmodulin (CaM) dependent kinase: CaM kinase II or CaMKII is a Ser/Thr-specific protein kinase involved in many signaling processes and is thought to be an important mediator of learning and memory. Ca2+ calmodulin complex regulates the enzyme activity. | ||
| 5 | Mn2+ | Arginase: This enzyme is required in the Urea cycle to disposes of the harmful ammonia from the body. It is the last enzyme in the urea cycle that hydrolysis the arginine and produces ornithine and urea. |
| Water oxidation complex of PS-II: This complex is also known as a water-plastoquinone oxidoreductase. That captures photons of light and converts them to energize electrons which are further transferred through a variety of coenzymes and cofactors to reduce plastoquinone to plastoquinol. Finally, it produces energy-rich molecules through the reduction process. | ||
| 6 | Ni 2+ | Urease: The plants, bacteria, and fungi produced urease, that hydrolyzes urea to ammonia and carbon dioxide. |
| 7 | K+ | Pyruvate kinase: Pyruvate kinase is the enzyme of glycolysis final step and it uses ADP and converts phosphoenolpyruvate to pyruvate to produces energy-rich molecule ATP. |
| 8 | Fe 3+/Fe2+ | Cytochromes: Cytochromes are heme-bonded proteins and they are three types such as cytochromes a, cytochromes b, and cytochromes. Electron transfer to another molecule is the main function of this protein in many metabolic pathways, especially cellular respiration. |
| Cis-aconitase: Cis-aconitase enzyme can be found in the citric acid cycle and that catalyzes citrate to isocitrate. | ||
| Tryptophan pyrrolase: This enzyme is also known as Tryptophan oxygenase. That is the heme-dependent liver cytosol enzyme that catalyzes the oxidative cleavage of the pyrrole ring of L- tryptophan to N-formyl kynurenine in the kynurenine-nicotinic acid pathway of tryptophan degradation. | ||
| Catalase: The universal enzyme Catalase is found in nearly all organisms such as bacteria, plants, and animals that are exposed to oxygen. It converts hydrogen peroxide (H2O2) to water and oxygen. Catalase plays a major role in protecting the cell from oxidative damage by reactive oxygen species (ROS). | ||
| Dinitrogenase complex: These enzymes are produced by cyanobacteria and responsible for the reduction of nitrogen to ammonia. | ||
| 9 | Cu2+/ Cu+ | Cytochrome oxidase: Cytochrome c oxidase is a large transmembrane protein complex found in mitochondria of eukaryotes and the membrane of bacteria and archaea. It is the last enzyme in the electron transport system and it undergoes oxidation and reduction process without binding of oxygen. It forms a water molecule by transferring electrons from ferrocytochrome c to molecular oxygen, this process is crucial to protect the cell from ROS damage. Cyanide inhibits the activity of this enzyme and leads to cell death. |
| Ceruloplasmin (Iron oxidase): Ceruloplasmin is an oxidase enzyme, normally transports iron elements from cells to plasma. The deficiency of ceruloplasmin leads to the abnormal accumulation of iron in cells of the pancreas, liver, retina, and the basal ganglia region of the brain. In blood circulation, the majority of copper is carried throughout the body by ceruloplasmin. In ceruloplasmin deficiency conditions copper will be excreted through urine. | ||
| Tyrosinase: Tyrosinase can be found in both plant and animal tissues, which play a major in the production of melanin and other pigments by oxidation of tyrosine. The black color formation of a peeled or sliced potato when exposed to air, is due to the oxidation of tyrosine. It also presents in melanosomes which are synthesized in the skin melanocytes. | ||
| Polyphenol oxidase: The enzyme polyphenol oxidase (PPO) present in plants and oxidizes the phenolic compounds into highly reactive quinones. Polymerization of PPO-derived quinones causes the postharvest browning of cut or bruised fruit. | ||
| 10 | V | Alternate nitrogenase: This is also known as vanadium nitrogenase produced by bacteria Azotobacter vinelandii. The enzyme involves in nitrogen fixation and used as an alternative to molybdenum nitrogenase. If molybdenum is unavailable then molybdenum nitrogenase will be inactive, in that case, microorganisms use vanadium nitrogenases for nitrogen fixation. |