Q. If two covalently bonded atoms are identical the bond is?
1. Polar Covalent bond
2. Nonpolar Covalent bond
3. Dipolar bond
4. Nonionic bond
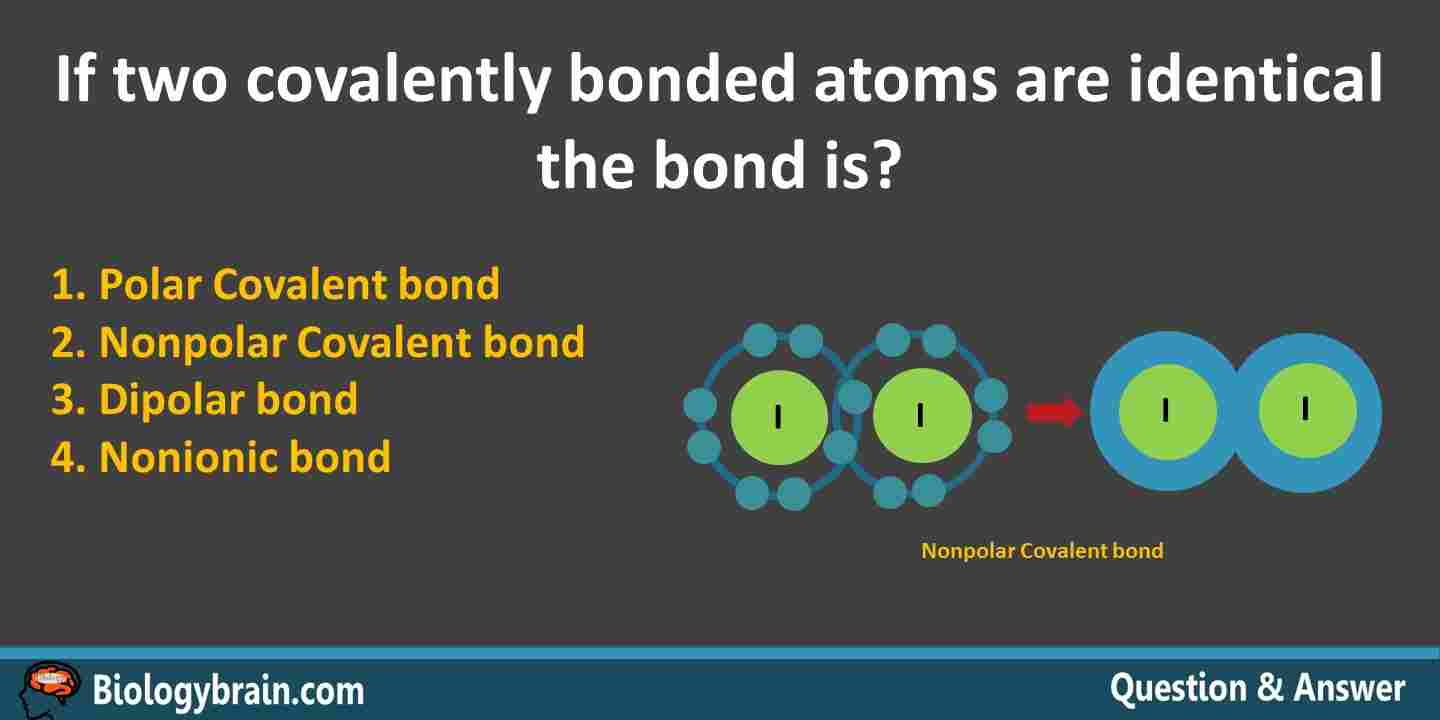
Answer: 2 (Nonpolar Covalent bond).
Explanation: If two covalently bonded atoms are identical (eg. Iodine) , then the bond is a nonpolar covalent bond. Two iodine atoms (See the above figure) exhibit equal share of valence electrons (equal electron density); hence, the bond is non polar. If the electron-sharing atoms show an unequal attraction for the electrons, then the bond between these two atoms is a polar covalent bond.
Other important questions:
Q. The alpha-helix and beta-sheet are found at which level of protein organization?
Q. Which cytoskeletal proteins provide the structural support for microvilli?
Q. Which of these does not contain a structural protein?
Q. What level of protein structure is associated with the sequence of amino acids?
Q. Which of the following pertains to typhoid fever?
Q. Which of the following tests is an agglutination test for the bacterium causing typhoid fever?
Q. This is a compound made from a group of covalently bonded atoms?