Definition of lysosomal storage disease
The lack of functional enzymes to regulate the excessive accumulation of their respective substrates has led to the emergence of genetic conditions known as lysosomal storage diseases.
When a lysosomal enzyme is absent or only partially functional, the corresponding materials tend to aggregate, which contributes to lysosomal storage disorders.
Only under autosomal recessive circumstances, which require that both parents of the fetus have a defective gene that prevents the cells from producing functional enzymes or proteins, do lysosomal storage disorders develop as hereditary conditions.
The following is a list of some of the main lysosomal disorders, their signs and symptoms, and the defective genes that cause them.
Key points of Lysosomal storage diseases
- Both autophagy and lysosomal degradation are two different types of proteolytic mechanisms that are present in every eukaryotic cell.
- The autophagy mechanism eliminates particular cell organelles, such as mitochondria, endoplasmic reticulum (ER), peroxisomes, and the Golgi complex, which are designated as undesirable or aging factors.
- Lysosomes are frequently referred to as the cell’s “recycle bins” and “suicidal bags.”
- The cellular or misfolded proteins that have accumulated and become unwanted are degraded by lysosomes using their own enzymes.
- Lysosomal storage disease develops when there are insufficient lysosomal enzymes in the lysosomes.
- The different lysosomal storage conditions have been classified according to the specific enzyme deficiency.
- The loss of function of a particular gene within the genome is the cause of the lack of particular enzymes or enzymatic activity.
- Lysosomal storage diseases are autosomal recessive, which means both parents of the affected child passed a mutated gene to their child.
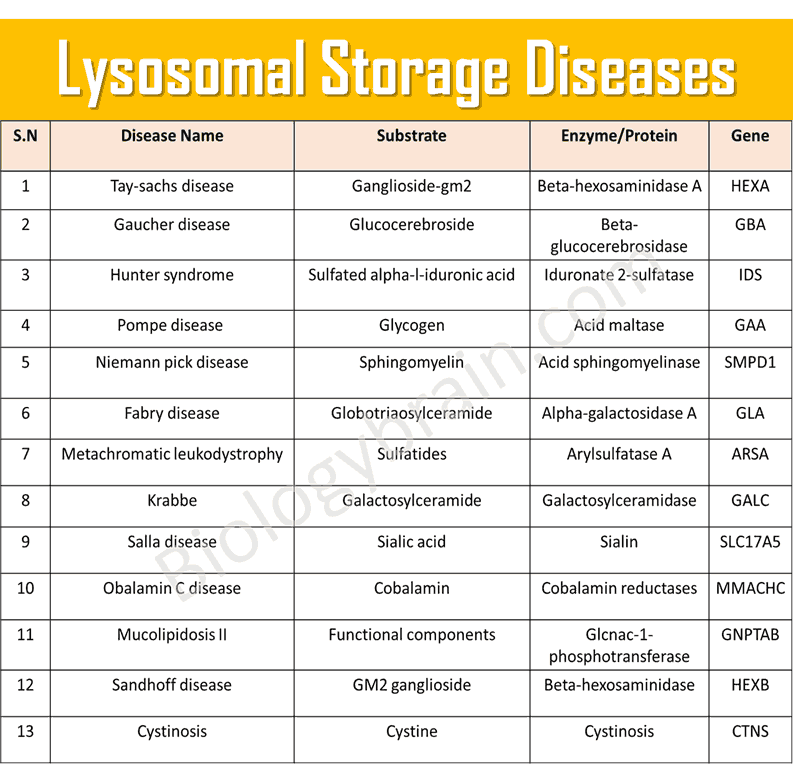
The list of major lysosomal storage diseases
- Tay-Sachs disease
- Gaucher disease
- Hunter syndrome
- Pompe disease
- Niemann Pick disease
- Fabry disease
- Metachromatic leukodystrophy
- Krabbe
- Cobalamin C disease
- Salla disease (free sialic acid storage disease)
- Mucolipidosis II
- Sandhoff disease
- Cystinosis
1. Tay-Sachs disease
A dangerous genetic condition called Tay-Sachs is inherited from one’s parents. An autosomal recessive disease called Tay-Sachs slowly kills the neurons in the spinal cord and brain. This condition is brought on by the absence of the HEXA gene, which codes for the lysosomal enzyme beta-hexosaminidase A, and is necessary for its synthesis. The lysosomes are home to this enzyme, which is essential for the brain and spinal cord. In the lysosomes, a fatty substance known as ganglioside-GM2 is generally broken down by beta-hexosaminidase-A.
Ganglioside-GM2 builds up because there aren’t any active enzymes. Particularly in the spinal cord and brain’s neurons, the buildup of GM2 substances acts as a toxic substance. GM2-gangliosidosis or lysosomal storage disorder are other terms used to describe this condition. Tay-Sachs disease is associated with a number of birth defects, including paralysis, blindness, hearing loss, intellectual disability, and seizures.
2. Gaucher disease
Gaucher disease is an inherited condition that is attained from parents with defective genes. The majority of body tissues and organs are impacted by this lysosomal storage disorder. Gaucher disease has been classified into several types based on the characteristics of the disease, including Cardiovascular Type Gaucher Disease, Perinatal Lethal Gaucher Disease, and Type-1 Gaucher disease, Types 2 and 3. The enzyme beta-glucocerebrosidase is a crucial component of lysosomes.
In physiological conditions, the enzyme beta-glucocerebrosidase transforms the substrate glucocerebroside into glucose and a trace amount of a fat molecule called ceramide. The gene known as GBA produces this functional enzyme in general conditions. Glucocerebrosides build up as a result of any GBA gene mutations that cause the abnormal or dysfunctional enzyme to be produced. The cells are more prone to excessive accumulation of the appropriate substrate, which can accumulate to higher toxic levels within the cells, when beta-glucocerebrosidase activity is diminished or eliminated. Different body organs may suffer damage as a result of these toxic levels.
a. Type-1 Gaucher disease
Type-1 Gaucher disease Type 1 is also referred to as non-neuronopathic Gaucher disease Type-1 since it could not harm nerve cells in the brain or spinal cord. Hepatosplenomegaly, a condition where the liver and spleen are enlarged, and anemia, thrombocytopenia, lung disease, and abnormalities in bone formation are some of the main signs and symptoms of this type of illness.
b. Gaucher disease type 2 and 3
The other diseases, such as Type 2 and Type 3, can seriously harm the nervous system and lead to potentially fatal conditions. Therefore, Gaucher disease types 2 and 3 are referred to as neuronopathic diseases. Struggle with eye movement, seizures, and brain damage are the main manifestations of these diseases.
c. Perinatal lethal Gaucher disease
The early stages of a baby’s development while still inside the womb, or before birth (in infancy), are when this type of Gaucher disease first manifests. Perinatal Lethal Gaucher disease is the name given to the most severe form of the illness. Hepatosplenomegaly, idiosyncratic facial features, severe neurological disorders, and ichthyosis (dry, scaly skin) are the main characteristics of the perinatal lethal disease. Hydrops fetalis is an extensive swelling brought on by fluid accumulation before birth. Due to these serious symptoms, the infant may pass away after birth or, in a few rare instances, even before it is born.
d. Cardiovascular type Gaucher disease
The heart is affected by this variant of Gaucher disease, which causes the heart valves to harden (calcify). Cardiovascular Gaucher disease patients may also experience other conditions like vision abnormalities, bone disease, and mild spleen enlargement (splenomegaly).
3. Hunter syndrome
Mucopolysaccharidosis type II (MPS II), a lysosomal storage disorder, also known as Hunter syndrome that primarily affects men and presents with a variety of physical symptoms. Although the rate of progression will vary among affected people, this disease is referred to as a disorder that worsens over time.
Iduronate 2-sulfatase, an enzyme that is present in lysosomes, is produced by the gene IDS. This enzyme is required for the breakdown of the large sugar molecules known as glycosaminoglycans (GAGs). Heparan and dermatan sulfates are two different types of glycosaminoglycans that contain the molecule known as sulfated alpha-L-iduronic acid, which is separated from the sulfate group by the enzyme iduronate 2-sulfatase. This faulty enzyme causes excessive glycosaminoglycan aggregation in the cell, which is related to mutations in IDS and causes this aggregation. The lysosomes’ size is increased due to the high concentration of glycosaminoglycans there, which causes tissues and organs to enlarge.
Typically, the disease affects children between the ages of 2 and 4; these kids develop macroglossia, which is characterized by broad noses, large, rounded cheeks, large lips, and an enlarged tongue.

4. Pompe disease
Glycogenosis Type II, GSD II, GSD2, AMD deficiency of alpha-glucosidase, GAA deficiency, glycogen lysosomal storage disease type II, alpha-1,4-glucosidase deficiency disorder, and Pompe’s disease are additional names for this condition.
In cells of the body, a complex sugar molecule known as glycogen aggregates excessively in Pompe disease, an inherited genetic autosomal recessive disorder.
Muscular function abnormalities are brought on by glycogen buildup in the organs or tissues, particularly the muscles. Acid alpha-glucosidase, also referred to as acid maltase, is a lysosomal enzyme produced by the GAA gene. Glycogen builds up excessively as a result of any mutation in this gene that results in an insufficient amount of the enzyme or the production of the enzyme’s inactive form. This enzyme is essential for the conversion of glycogen into the usable energy molecule glucose in lysosomes.
According to research, the three types of Pompe disease—infantile-onset, non-classical infantile-onset, and late-onset—are distinguished by their severity and age at which they manifest.
a. Infantile-onset
Within a few months of birth, this form of Pompe lysosomal storage condition develops. Heart defects, muscle weakness (myopathy), poor muscle tone (hypotonia), and enlarged liver (hepatomegaly) are just a few of the health issues that infants with this type of disease may experience. Following the disease’s onset, infants may experience physical conditions like slow growth and trouble gaining weight. Infants who pass away too soon from heart failure in the first year of life are linked to untreated disease-related conditions.
b. Non-classic infantile-onset
Non-classical infantile-onset Pompe disease typically doesn’t exhibit symptoms until one year of age. It is linked to conditions like severely weakened muscles and delayed motor skills (like rolling over and sitting). Although the heart enlarges (cardiomegaly), the affected person typically does not experience any issues with heart function. However, most children with non-classical infantile-onset Pompe disease only live for a few years due to the muscle weakness that results from this disorder.
c. late-onset
Following adolescence or adulthood, the late-onset Pompe disease will manifest. But compared to infantile onset, the disease’s effectiveness is very low, and heart function is only infrequently affected. The majority of individuals with late-onset Pompe disease may experience issues like advancing muscle weakness, especially in the legs and trunk. This illness primarily affects the lungs, making breathing difficult. Complete respiratory failure can result from breathing problems that get progressively worse.
5. Niemann Pick disease
Numerous organs of the body are impacted by Niemann Pick, a lysosomal storage disease. When the lysosomal enzyme acid sphingomyelinase, which is necessary for the breakdown of excessively accumulated sphingomyelin in the lysosomes, is absent from birth, Niemann Pick disease manifests as being significantly more severe in those affected. Sphingomyelin, a larger lipid, is broken down into ceramide, a smaller lipid, by the active sphingomyelinase enzyme.
The SMPD1 gene is in charge of making the active sphingomyelinase enzyme, but any mutations in this gene cause an overproduction of this big molecule inside the lysosomes. This lipid molecule aggregates within cells, causing cell dysfunction and eventual cell death. The cells in tissues and organs, such as the brain, lungs, spleen, and liver, completely stop functioning as the disorder worsens.
Based on their genetic origin, the signs and symptoms of the condition, severity, and the age at which they manifest, the four main types of Niemann Pick disease—types A, B, C1, and C2—have been identified.
- Niemann Pick disease types A and B are brought on by a deficiency in the sphingomyelinase enzyme, which converts sphingomyelin to ceramide.
- Niemann Pick disease types C1 and C2 are brought on by a deficiency in functional proteins, which are necessary for the movement of lipids within cells.
6. Fabry disease
Lysosomal storage disorders such as Fabry disease can affect various body organs. The affected individuals appear to have severe cases of Fabry disease. The lysosome-located enzyme alpha-galactosidase A participates in the cellular breakdown of globotriaosylceramide.
The structure and activity of the enzyme are hampered by mutations in the GLA gene. The accumulation of the harmful substrate throughout the body is a symptom of the enzyme deficiency, which is also related to the absence of the cell’s breakdown process. Particularly, the kidney, heart, and nervous system cells as well as the skin’s blood vessels and cells lining them are significantly filled with this substrate. The more concentrated a substance is, the more damage it causes to cells, leading to Fabry disease’s various signs and symptoms.
Additionally, Fabry disease has been linked to conditions that could be fatal, including chronic kidney disease, heart failure, and frequent strokes.
A small percentage of those affected will have milder forms of the disorder that may develop later in life and only affect the heart or kidneys.
7. Metachromatic leukodystrophy
The lysosomal storage disorder known as metachromatic leukodystrophy is brought on by a gene mutation or by the absence of a functional gene that codes for the arylsulfatase A enzyme. The lysosome houses the enzyme arylsulfatase A. This enzyme helps break down sulfatides inside the lysosomes.
Genome mutations are found in those who have metachromatic leukodystrophy. This enzyme, which prevents an excessive accumulation of the substrate sulfatides, is specifically encoded by the gene ARSA. If this gene is altered, no useful enzyme can be produced. The nervous system is harmed by an excessive buildup of sulfatides in the cells, particularly in nerve cells.
Myelin substance, which insulates and shields nerves, is typically produced by nervous system cells. The entire nervous system is affected by the leukodystrophy (progressive destruction of white matter) caused by sulfatide aggregation in myelin-producing cells. The neurological issues are the primary symptoms of metachromatic leukodystrophy and are associated with peripheral neuropathy, loss of speech, seizures, paralysis, blindness, and hearing loss. The affected person may eventually lose awareness of their surroundings and stop responding.
8. Krabbe
An inherited disorder called Krabbe (pronounced “krah-buh”), also known as lysosomal storage disease, damages the myelin sheath that surrounds nerve cells in the brain and other parts of the nervous system. Krabbe disease typically manifests as late-onset forms in adults, but it can also occur in children, adolescents, and younger people.
In the early stages of the disorder, vision loss and difficulty walking are the main symptoms. However, individuals with the condition may experience various signs and symptoms. People with late-onset Krabbe disease have a best-case scenario of many years of survival. Krabbe disease is a severe condition that affects about 1 in 100,000 people in the US. Occasionally, it will be 6 out of 1000 people.
Galactolipids, also known as galactosylceramides, are among the fats associated with galactose that are broken down by the enzyme galactosylceramidase, which is produced in normal people by the gene GALC. The breakdown of galactosylceramide is a critical process because the normal turnover of myelin is required throughout life, as this lipid molecule is a key component of myelin.
Changes in the GALC gene prevent the production of a functional galactosylceramidase enzyme, which causes galactosylceramide to build up in some cells, e.g., forming globoid cells. The enzyme aggregates spoil the myelin synthesizing cells. The nervous system becomes demyelinated as a result of damaged cells’ inability to produce myelin. Krabbe disease is characterized by vision loss and difficulty walking because nerve cells in the brain and other parts of the body that lack a myelin sheet are unable to transmit signals correctly.
9. Salla disease (free sialic acid storage disease)
The most mild version of the free sialic acid lysosomal storage condition, known as Salla disease, primarily affects the nervous system. During the first year of life, infants with Salla disease typically experience hypotonia, or reduced muscle tone, which is followed by ongoing neurological issues.
Intellectual disability, developmental delay, seizures, speech impairment, ataxia, muscle spasticity, and uncontrollable slow limb movements (athetosis) are some of the disease’s signs and symptoms. But the majority of impacted kids eventually learn to walk. This condition is inherited autosomally recessively (both parents’ genes have an impact on the child) and is caused by mutations in the SLC17A5 gene. Commonly, symptomatic and supportive treatments are used to control the severity of the disorder.
A protein called sialin, which is primarily found on the membrane of the lysosome, a significant organelle in the cell that digests and recycles cellular components, is encoded by the SLC17A5 gene. Sialic acid, a metabolite produced during the digestion of some proteins and fats, is transported by sialin. Free sialic acid typically needs to move from the lysosomes to other areas of the cell. However, gene mutations result in an excessive buildup of free sialic acid in lysosomes, which causes the above-mentioned emerging serious health issues.
10. Cobalamin C disease
This lysosomal storage condition, also referred to as cobalamin C disease or methylmalonic aciduria with homocystinuria, is an inherited genetic condition. With advancing age, the disease’s onset and severity vary. Most people experience the first signs of a disease within the first year, which are frequently brought on by fasting, illness, infection, or eating more protein.
Cognitive impairment, hypotonia, lethargy, intellectual disability, seizures, and eye issues are some of the primary identifying symptoms. Vitamin B12, also referred to as cobalamin, is converted by the cobalamin reductases enzyme into either adenosylcobalamin (AdoCbl) or methylcobalamin (MeCbl), depending on whether it is present in the body. This process requires information from the MMACHC gene.
Methylmalonyl CoA mutase needs adenosylcobalamin in order to operate normally. The enzyme breaks down a few of the amino acids that make up proteins as well as lipids, which include cholesterol, and fats. For methylmalonyl CoA mutase, adenosylcobalamin is referred to as a cofactor because it aids in the activity of the enzyme. Methionine synthase also needs the cofactor adenosylcobalamin to function. This enzyme changes the amino acid homocysteine into methionine. Methionine, an amino acid, is used by the body to create proteins and other vital compounds that are methionine-derived.
Mutations in the MMACHC gene cause a buildup of these substances and their metabolites in the body’s organs and tissues, which is what causes the disease’s newly appearing symptoms.
11. Mucolipidosis II
An inherited lysosomal storage disorder called mucolipidosis II continuously harms various body parts. Most often, those who are affected won’t live very long. A newborn’s life may end unexpectedly early. During the first few years of life, the affected child begins to exhibit symptoms such as hypotonia and a feeble cry. Children who are affected will gradually lose their ability to grow after their second year of life and will stop growing properly after birth. The growth of the body’s organs will be stunted, and the development of speech and motor abilities will be hampered.
Children who are affected typically have clubfeet, dislocated hips, abnormally rounded upper backs (kyphosis), long bones with unusual shapes, and short hands and fingers. Additionally, the baby who has this condition has joint contractures that significantly limit mobility. In most cases, infants who develop mucolipidosis II are unable to walk on their own.
Mutations and other gene modulatory factors alter the frequency of the gene, resulting in loss of function of the corresponding gene, which is the primary cause of this disorder in developing babies. The functional enzyme GlcNAc-1-phosphotransferase, which transports some newly synthesized enzymes to lysosomes, is no longer produced by the inactive gene GNPTAB. Overall, certain lysosomal storage conditions are brought on by lysosomes that lack these functional components.
12. Sandhoff disease
An inherited lysosomal storage disorder called Sandhoff results in the accumulation of unwanted materials like fats and sugars in the lysosomes, which kills nerve cells (neurons) in the brain and spinal cord. Although Sandhoff disorder is a rare disease in humans, the most prevalent and severe form first manifests in infancy. Until they are 3 to 6 months old, babies who are prone to this lysosomal storage disorder present normally. As soon as they begin to develop, the consequences will become apparent, slowing the growth and limiting muscle movement.
Babies who are affected also experience vision, hearing, and motor skill loss. The primary distinguishing feature of this eye disorder, known as the Cherry-red spot, is detectable with an eye examination.
The specific protein, which is a component of the two significant lysosomal enzymes in the nervous cells, beta-hexosaminidase A and beta-hexosaminidase B, is produced by the functional gene HEXB in living cells. Beta-hexosaminidase A and beta-hexosaminidase B are inhibited from cleaving GM2 ganglioside and other molecules by any mutation in the HEXB gene, which also removes the activity of these enzymes.
Due to the presence of faulty enzymes or a lack of enzymes, GM2 ganglioside, one of their respective substrates, accumulates too much. Particularly in the brain and spinal cord’s nerve cells, this condition causes an accumulation of unwanted substrate at toxic levels. Many of the signs and symptoms of Sandhoff disease and other lysosomal storage disorders (like gangliosidosis), as well as other lysosomal storage disorders, such as GM2 ganglioside buildup, are caused by the ongoing damage of nerve cells.
13. Cystinosis
An excessive buildup of the amino acid cystine inside of cells results in cystinosis, a lysosomal storage disorder that is inherited. Cysteine in toxic concentrations damages cells and frequently forms crystals that can accumulate and cause issues in different organs and tissues. Particularly vulnerable to harm are the kidneys and eyes, but other organs like the muscles, pancreas, thyroid, and testes may also be impacted. There are three types of cystinosis, categorized according to severity: nephropathic cystinosis, intermediate cystinosis, and non-nephropathic or ocular cystinosis.
The most severe form of nephropathic cystinosis, which affects newborns and results in stunted growth and various kidney issues, is present. The conditions that are most likely to be the cause of symptoms include hypophosphatemic rickets, increased urination, thirst, dehydration, abnormally acidic blood, photophobia, muscle deterioration, blindness, inability to swallow, diabetes, thyroid, and nervous system issues.
A moderate-to-severe form of cystinosis, intermediate cystinosis can occasionally have a high severity and be brought on at a later age. Most affected individuals with intermediate cystinosis first show symptoms during adolescence. Crystals in the cornea and damaged kidneys are the primary initial defining characteristics of this disorder. Although this usually takes place in late adolescence or the middle of adolescence, untreated intermediate cystinosis can completely destroy the kidney.
Comparing the three forms, non-nephropathic is a little less severe than the other two. Non-nephropathic or ocular cystinosis typically does not result in kidney problems or the majority of the other signs and symptoms of cystinosis, with the exception of photophobia caused by cystine crystals in the cornea. The age at which this type of cystinosis is diagnosed distinctly varies due to the lack of severe symptoms.
Mutations in the CTNS gene are the basis for these three types of cystinosis. Any gene mutation leads to a lack of a transporter protein called cystinosis. This seven-transmembrane protein, which serves as an active transporter within cells, typically transports cystine and amino acids from lysosomes, which are organelles in the cell that digest and recycle materials. When cystinosis is inadequate or flawed, cystine builds up in the lysosomes in toxic amounts and crystallizes. Other organs may also be impacted by these cystine aggregates, which can also harm kidney and eye cells.
Frequently asked questions
1. Which of the following cell can release the enzymes of the lysosome to the outside of the cell to digest the extracellular components?
a) Osteoblast
b) Osteoclast
c) Osteocytes
d) Hepatocytes
Answer: The bone resorptive cell such as osteoclast is involved in extracellular digestion by releasing the enzymes of lysosomes outside the cell.
2. Which of the following lysosomal storage disease causes severe dame to neurons of the brain and spinal cord (central nervous system), which is similar to the conditions and symptoms of Tay-Sachs disease.
a) Gaucher disease Type-1 and 2
b) Gaucher disease Type-2 only
c) Gaucher disease Type-1 and 3
d) Gaucher disease Type-2 and 3
Answer: Gaucher disease Type-2 and 3
References
- https://ghr.nlm.nih.gov/condition/gaucher-disease#sourcesforpage.
- https://ghr.nlm.nih.gov/condition/niemann-pick-disease#sourcesforpage.
- https://ghr.nlm.nih.gov/condition/mucopolysaccharidosis-type-ii#sourcesforpage.
- https://ghr.nlm.nih.gov/condition/fabry-disease#
- https://ghr.nlm.nih.gov/condition/metachromatic-leukodystrophy#resources.
- Lysosomal storage disease NCBI.
- A lysosomal storage disease involving inflammation. 285
- Sequelae of storage in Fabry disease–pathology and comparison with other lysosomal storage diseases,
- A new lysosomal storage disease, Arch Neurol 36(2) (1979), 88–94.
- An acquired lysosomal storage disease, Biochim Biophys Acta1831(3) (2013), 602–611
- Newborn screening for lysosomal storage diseases, Clin Chem61(2) (2015), 335–346.
- Lysosomal storage disease screening implementation: J Pediatr166(1) (2015), 172–177.
- Neonatal screening for lysosomal storage disease: 335–341.
- Lysosomal storage disorders: The cell biology of disease.
- Nina Aula,Pertti Aula, 2006. Prenatal diagnosis of free sialic acid storage disorders (SASD)-(https://obgyn.onlinelibrary.wiley.com/doi/10.1002/pd.1431).
- Pompe disease-MedlinePlus (https://medlineplus.gov/genetics/condition/pompe-disease/)
- Cystinosis-MedlinePlus (https://medlineplus.gov/genetics/condition/cystinosis/#description)
- Ferreira, Carlos R., and Gahl, William. Lysosomal storage diseases. Translational Science of Rare Diseases, vol. 2, no. 1-2, pp. 1-71, 2017.
- K Wyatt, W Henley, L Anderson, R Anderson, et al., 2012. The effectiveness and cost of enzyme replacement and substrate reduction therapies: a longitudinal cohort study of people with lysosomal storage disorders. Health Technology Assessment,16:39.
- Deborah Elstein, Gheona Altarescu, Michael Beck. Fabry Disease, 2010. (https://link.springer.com/book/10.1007/978-90-481-9033-1)
- Institute Of Immunity And Transplantation.(www.ucl.ac.uk/immunity-transplantation/clinical-services/diseases-treatments/inherited-diseases/lysosomal-storage-disease)